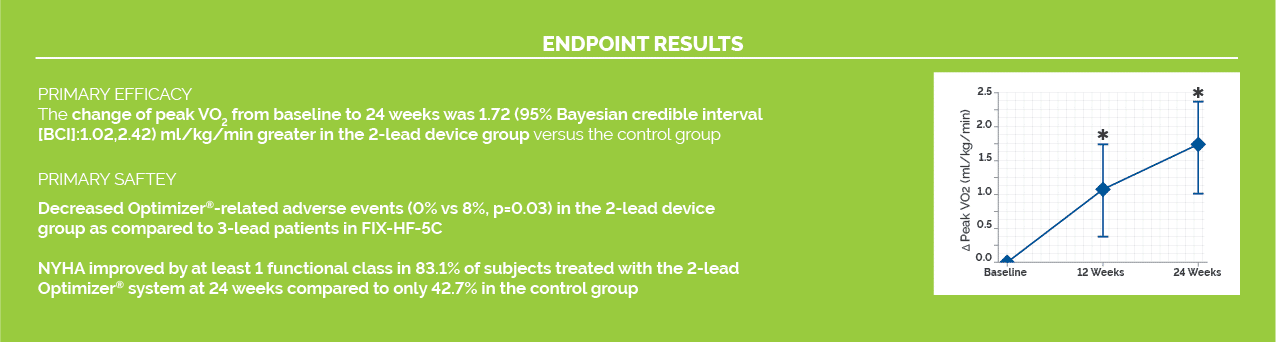
FIX-HF-5C2 STUDY

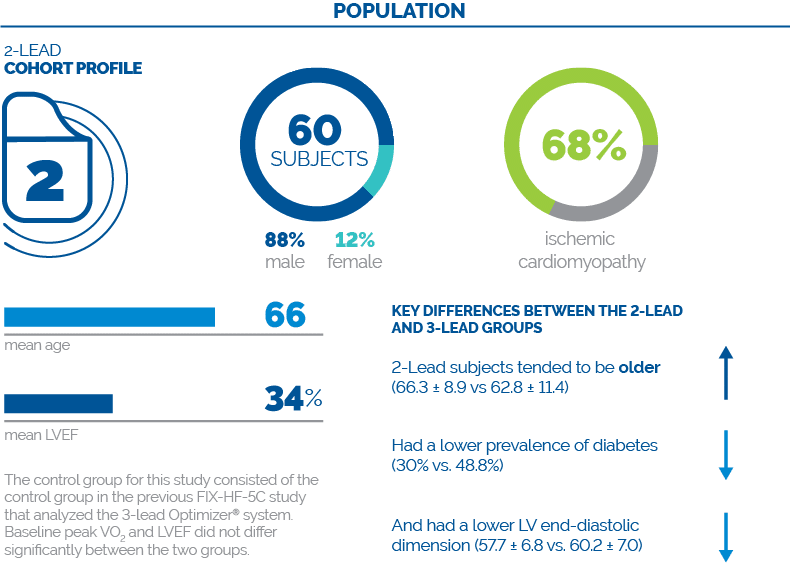
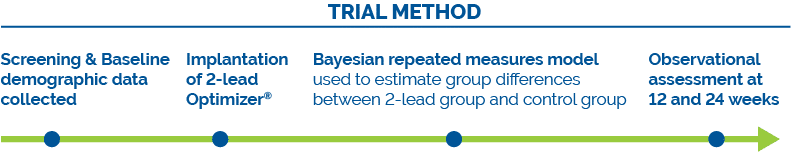

The main limitation of the present study is that it was a nonrandomized, unblinded study that used a historical control group from the prior FIX-HF-5C study. The two studies are reasonably contemporaneous, having been completed less than 2 years of each other. The only significant difference in background medical therapy was a slightly greater use of valsartan/sacubitril in the current study (15% vs 4%) due to its introduction into clinical practice towards the completion of enrollment into the FIX-HF-5C study.

© 2025 Impulse Dynamics Terms of Use | Data Protection | Vulnerability Disclosure | Terms and Conditions | Privacy Policy