Back to Clinicals Page

Test the performance, safety and clinical effects of a 2-lead CCM® device vs a 3-lead CCM® device

The 2-lead system effectively delivers comparable amount of CCM® pulses as the 3-lead system, is equally safe and improves peak VO2 and NYHA functional class. Device-related adverse effects are less with the 2-lead system.
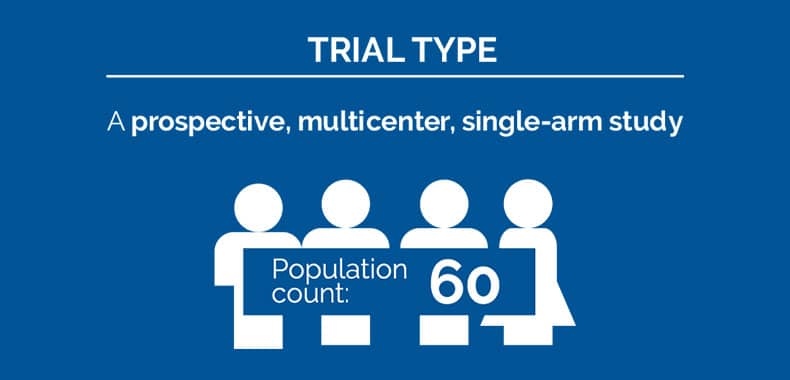
A prospective, multicenter, single-arm study
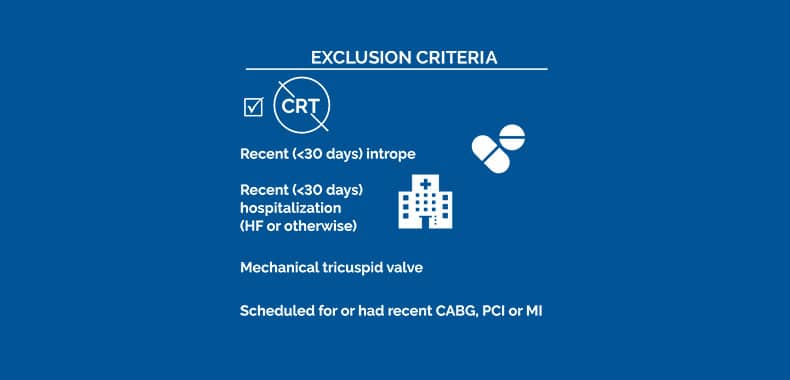
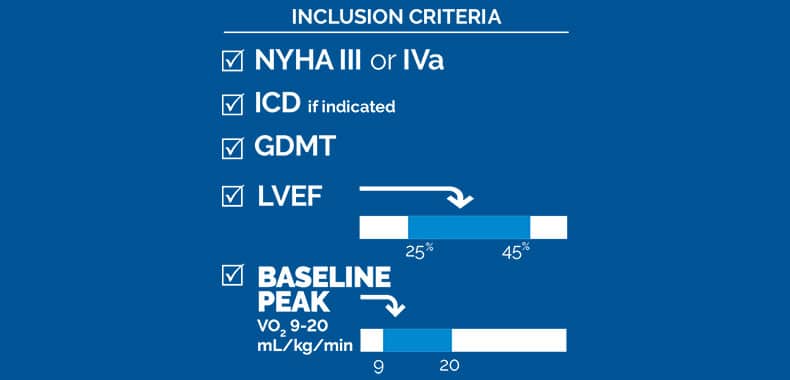
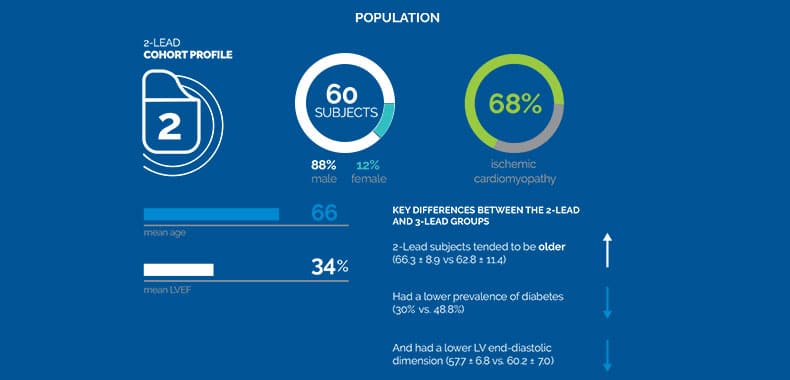
The control group for this study consisted of the control group in the previous FIX-HF-5C study that analyzed the 3-lead Optimizer® system. Baseline peak VO2 and LVEF did not differ significantly between the two groups.
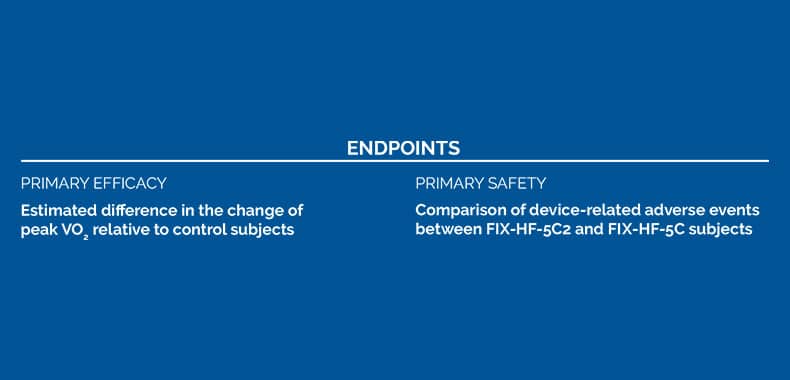
Endpoints
PRIMARY EFFICACY
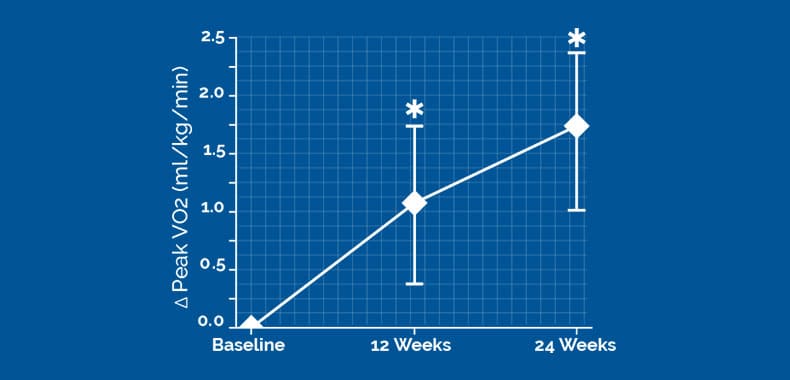
The change of peak VO2 from baseline to 24 weeks was 1.72 (95% Bayesian credible interval [BCI]:1.02,2.42) ml/kg/min greater in the 2-lead device group versus the control group
PRIMARY SAFTEY
Decreased Optimizer®-related adverse events (0% vs 8%, p=0.03) in the 2-lead device group as compared to 3-lead patients in FIX-HF-5C
NYHA improved by at least 1 functional class in 83.1% of subjects treated with the 2-lead Optimizer® system at 24 weeks compared to only 42.7% in the control group
ABSTRACT
Background: Prior studies of cardiac contractility modulation (CCM) employed a 3-lead Optimizer system. A new 2-lead
system eliminated the need for an atrial lead. This study tested the safety and effectiveness of this 2-lead system compared
with the 3-lead system.
Methods: Patients with New York Heart Association III/IVa symptoms despite medical therapy, left ventricular ejection
fraction 25% to 45%, and not eligible for cardiac resynchronization therapy could participate. All subjects received an
Optimizer 2-lead implant. The primary end point was the estimated difference in the change of peak VO2 from baseline to 24
weeks between FIX-HF-5C2 (2-lead system) subjects relative to control subjects from the prior FIX-HF-5C (3-lead system)
study. Changes in New York Heart Association were a secondary end point. The primary safety end point was a comparison
of device-related adverse events between FIX-HF-5C2 and FIX-HF-5C subjects.
Results: Sixty subjects, 88% male, 66±9 years old with left ventricular ejection fraction 34±6% were included. Baseline characteristics
were similar between FIX-HF-5C and FIX-HF-5C2 subjects except that 15% of FIX-HF-5C2 subjects had permanent atrial
fibrillation versus 0% in FIX-HF-5C. CCM delivery did not differ significantly between 2- and 3-lead systems (19892±3472 versus
19583±4998 CCM signals/day, CI of difference [−1228 to 1847]). The change of peak VO2 from baseline to 24 weeks was 1.72 (95% Bayesian credible interval, 1.02–2.42) mL/kg per minute greater in the 2-lead device group versus controls. 83.1% of 2-lead subjects compared with 42.7% of controls experienced ≥1 class New York Heart Association improvement (P<0.001). There were Optimizer-related adverse events with the 2-lead system compared with the 3-lead system (0% versus 8%; P=0.03).
Conclusions:The 2-lead system effectively delivers comparable amount of CCM signals (including in subjects with atrial
fibrillation) as the 3-lead system, is equally safe and improves peak VO2 and New York Heart Association. Device-related
adverse effects are less with the 2-lead system.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT03339310

© 2024 Impulse Dynamics Terms of Use | Data Protection | Vulnerability Disclosure | Terms and Conditions